Flexible Binder for S@pPAN Cathode of Lithium Sulfur Battery- part one
Experimental method
1.1 Material preparation
Weigh a certain amount of polyacrylonitrile (Mw=1.5×105, Aldrich) and elemental sulfur according to the mass ratio of 1:8, add an appropriate amount of absolute ethanol as a dispersant, and mix them evenly in a sealed agate ball mill jar. After ball milling for 6 hours, it was dried in a blast oven at 60 °C. After drying, grind the block mixture well. Then a certain amount of mixed powder was weighed and placed in a quartz boat, and the temperature was raised to 300 °C in a tube furnace under a nitrogen protective atmosphere, and kept for 6.5 h to obtain a S@pPAN black powder with a sulfur mass fraction of 41%. Weigh 20 mg SWCNT into a sample bottle, and then add 0.5 mg·mL-1 sodium dodecylbenzenesulfonate (SDBS). After ultrasonic treatment for 10 hours, CMC (Mw=7×105, Aldrich) was added to the SWCNT suspension (the mass ratio of CMC and SWCNT was 2:1) and stirred for 2 hours to obtain SCMC, and its solid content mass fraction is 1%。In addition, the CMC used in the control experiment is exactly the same as the CMC used in the above SCMC synthesis without other treatment. Dissolve CMC in deionized water, the mass fraction of CMC is 1%, and the sample is labeled as CMCP.
1.2 Electrode preparation and battery assembly
S@pPAN, Super P and bonding slurry (SCMC or CMCP) were weighed according to the mass ratio of 8:1:1. Put it in a polytetrafluoroethylene tank for ball milling for 2 hours, and the mass of the bonded slurry is calculated according to the mass of the solid phase component. The slurry was coated on the carbon-coated aluminum foil with a film applicator, and after drying at room temperature, it was cut into ϕ12 mm discs with a microtome, and dried in a blast oven at 70 °C for 6 hours. After pre-drying, the pole piece was processed with a tablet press under a pressure of 12 MPa to reduce the thickness of the pole piece and increase the compaction density of the pole piece, and then continue to vacuum dry at 70 °C for 6 hours. After the temperature of the vacuum oven dropped to room temperature, the pole piece was quickly transferred to the glove box for weighing and set aside. The active material loading per unit area of the cathode in this study is about 0.6 mg∙cm-2. The electrodes based on SCMC and CMCP are denoted as S@pPAN/SCMC and S@pPAN/CMC, respectively.
1.3 Electrochemical performance test
A 2016-type button battery was assembled in the order of positive electrode case, positive electrode sheet, separator, and lithium sheet. The electrolyte is 1 mol L-1 LiPF6 ethylene carbonate (EC)/dimethyl carbonate (DMC) (volume ratio 1 : 1) solution + mass fraction 10% fluoroethylene carbonate (10% FEC), The diaphragm is a polyethylene (PE) diaphragm.Use the Xinwei battery test system to conduct constant current charge and discharge tests on the assembled batteries. The battery was allowed to stand for 4 h before cycling to fully infiltrate the separator and electrodes with the electrolyte. The charge-discharge cut-off voltage ranged from 1.0 to 3.0 V, and a constant temperature of 25 °C was maintained during cycling. The long-term cycle test was carried out at 2C current density, and the rate performance of the battery was tested at 0.5C, 1C, 3C, 5C, and 7C current density. Cyclic voltammetry (CV) was performed on a CHI 760E electrochemical workstation with a scan rate of 1 mV s-1. The specific capacity is calculated based on the active component sulfur.
1.4 Physical Properties Characterization
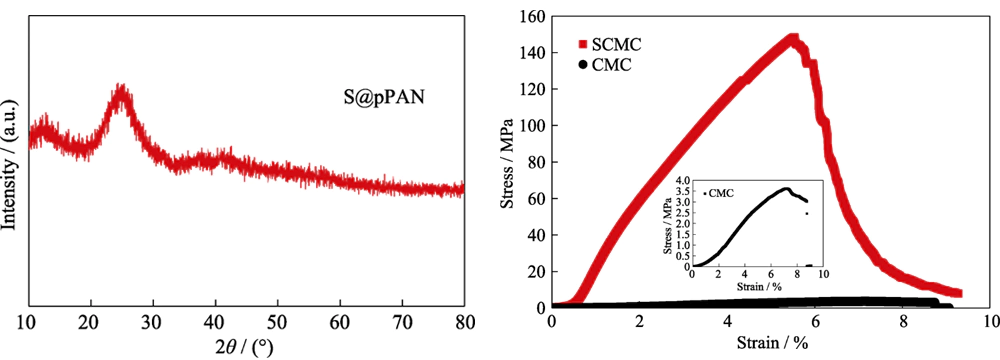
X-ray photoelectron spectroscopy (XPS) was used to analyze the surface elements of lithium sheets after battery cycling, and the sample preparation was completed in a glove box. The XRD spectrum of the S@pPAN material was tested by X-ray diffractometer (XRD).More Lithium ion Battery Materials from TOB New Energy