In recent years, the rapid development of sulfide solid electrolytes—including Li₂S-SiS₂, Li₂S-B₂S₃, Li₂S-P₂S₅, Li(₁₀±₁)MP₂S₁₂(where M = Ge, Si, Sn, Al, or P), and Li₆PS₅X (where X = Cl, Br, I)—has, in particular, partially addressed the drawback of insufficient intrinsic conductivity in solid electrolytes. This progress is exemplified by thio-LISICON-structured sulfides such as Li₁₀GeP₂S₁₂(LGPS), which exhibit an extremely high room-temperature lithium-ion conductivity of 12 mS/cm, surpassing that of liquid electrolytes.
Figure 1(a) shows an all-solid-state lithium battery using a cold-pressed pellet of Li₁₀Ge₂PS₁₂ceramic solid electrolyte powder with a room-temperature electrical conductivity exceeding 5 mS/cm, a LiCoO₂cathode material, a 99% (30Li₂S·70P₂S₅)·1% P₂O₅electrolyte as the anode-side modifier electrolyte, and metallic lithium as the anode. This battery can normally discharge and operate at room temperature to light up an LED lamp. A schematic diagram of the core component structure is shown in Figure 1(b), from which it can be seen that the cathode layer, inorganic solid electrolyte layer, and lithium foil are tightly bonded and pressed together in a mold. The preparation methods and processes of each component will be described in detail below.
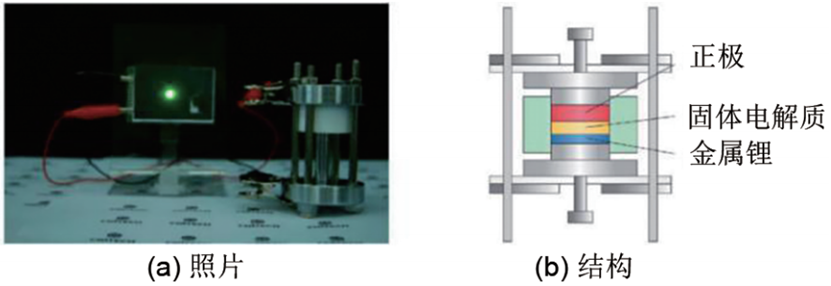
Figure 1: All-Solid-State Lithium Battery Based on Sulfide Solid Electrolyte
1 Preparation Method of the Cathode
Sulfide solid electrolyte powder exhibits a Young's modulus of approximately 20 GPa, along with strong adhesion, high compressibility, and a tendency for plastic deformation. After cold pressing, it demonstrates low grain boundary resistance; thus, it is suitable for direct dry mixing with cathode powder during the preparation of the cathode layer [Fig. 2(a)]. During dry mixing, the conductive agent, sulfide solid electrolyte, and cathode material are simultaneously added to a mortar, by manual grinding, or mechanically mixed using a stirrer. It should be noted that the compatibility between different cathode materials and the electrolyte, the applicability of various conductive agents, and the suitability of different cathode coatings need to be evaluated under practical conditions.
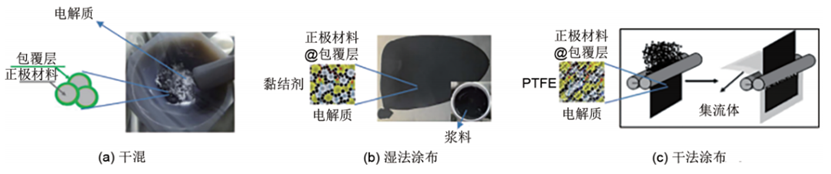
Figure 2: Preparation Method of the Cathode for All-Solid-State Lithium Batteries Based on Sulfide Solid Electrolytes
For large-scale roll-to-roll (R2R) fabrication of sulfide batteries, the wet coating process [Fig. 2(b)] may be more suitable for scaling up. This is because polymer binders and solvents are required to prepare thin-film electrolyte and electrode layers with the mechanical properties needed for high-throughput R2R processes. Additionally, the presence of flexible polymers in the electrolyte/electrode can effectively buffer the stress and strain generated during repeated charge-discharge cycles, mitigating issues such as crack formation and particle detachment.
However, the following considerations are necessary during preparation: ① The polymer binder should be dissolved in non-polar or weakly polar solvents (e.g., xylene) with negligible reactivity toward sulfides; ② Binders with strong adhesion should be used, as excessive polymer would adversely affect ionic conductivity and the thermal stability of the electrolyte/electrode; ③ The polymer binder must exhibit high flexibility. Although polymers such as polystyrene (PS) and poly(methyl methacrylate) (PMMA) can dissolve in xylene, they become extremely rigid after solvent evaporation, causing the electrolyte/electrode to crumble. Thus, most studies have selected nitrile rubber (NBR) and styrene-butadiene rubber (SBR). However, rubber-based binders cannot generate internal ionic conductivity, which significantly degrades the battery’s electrochemical performance even when used in small amounts. Therefore, developing polymers with high ionic conductivity, excellent thermal stability, solubility in non-polar or weakly polar solvents, and insolubility in polysulfides is the future direction for the wet coating of sulfide electrolytes.
Nonetheless, the wet slurry preparation process described above involves significant solvent usage, which inevitably leaves residual small-molecule solvents in the mixture. These residues can trigger side reactions, reducing electrolyte conductivity and severely degrading battery lifespan. Additionally, the incomplete encapsulation of active materials by solution-based polymer binders may lead to charge transfer failure. Solvent evaporation also results in low compactness of the electrode sheets, which hinders the battery’s kinetic processes. Furthermore, solvent emission and recovery pose unavoidable challenges for scaled-up production.
Thus, the dry coating technology using polytetrafluoroethylene (PTFE) [Fig. 2(c)] has emerged as an alternative approach. It primarily involves three steps: ① Dry-mixing the electrolyte, electrode materials, and PTFE via ball milling; ② Rolling the powder into a thin film; ③ Rolling the film with a current collector to form the electrode. Due to the extremely weak intermolecular forces between fluorine-carbon chains in PTFE and the high flexibility of its molecular chains, fine PTFE powder particles with large molecular weights undergo fibrosis under directional forces. Specifically, microparticles within the grains align regularly in the direction of shear force, forming fibrous and network structures. This enables the close yet non-complete encapsulation of active materials, electrolytes, and conductive carbon.
2 Preparation Method of the Anode
Ternary sulfide solid electrolytes with a thio-LISICON structure exhibit high
conductivity; however, experimental and computational studies have reported
that spontaneous and progressively propagating interfacial reactions between
metallic lithium and electrolytes such as LGPS or Li₁₀Sn₂PS₁₂generate interfacial phases with low ionic conductivity (e.g., Li₂S, Li₃P) and high electronic conductivity (e.g., Li₁₅Ge₄). These phases increase the Li/LGPS interfacial impedance, cause
short-circuiting in all-solid-state lithium batteries, and severely limit the
development of high-energy-density all-solid-state lithium batteries. To
enhance the chemical/electrochemical stability of sulfide
electrolytes—especially ternary sulfides containing germanium, tin, zinc,
etc.—toward metallic lithium, there are currently three main approaches.
(1) Surface treatment of metallic lithium to in-situ generate a surface ion-conductive modification layer for protecting the sulfide electrolyte. As shown in Fig. 3(a), Zhang et al. achieved an increase in the contact area between the modification layer and metallic lithium by controlling the reaction of Li with pure H₃PO₄to form an LiH₂PO₄protective layer. This avoided direct contact between metallic lithium and LGPS, prevented the penetration of mixed ionic-electronic conducting intermediate phases into the interior of LGPS, and improved the issue of sluggish interfacial lithium-ion kinetics. Results showed that with the LiH₂PO₄modification, the lithium stability of LGPS was significantly enhanced. The LCO/LGPS/LiH₂PO₄-Li all-solid-state lithium battery delivered ultra-long cycle life and high capacity: at 25°C and a 0.1C rate, the reversible discharge capacity of the 500th cycle remained at 113.7 mA·h/g, with a retention rate of 86.7%. Additionally, the Li/Li symmetric cell could stably cycle for over 950 hours under a current density of 0.1 mA/cm².
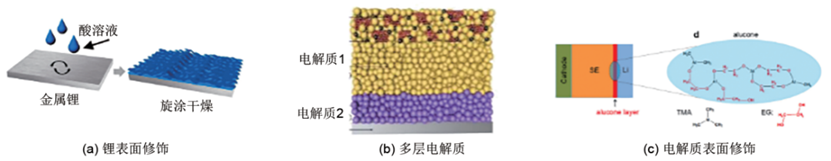
Figure 3: Anode Modification Method for All-Solid-State Lithium Batteries Based on Sulfide Solid Electrolytes
(2) Using a transition-layer sulfide solid electrolyte that is stable toward metallic lithium to protect the other layer. As shown in Fig. 3(b), Yao et al. proposed an LGPS/LPOS bilayer electrolyte structure to enhance the ionic conduction and stability of the LGPS/Li interface, which has achieved favorable results in various battery systems. However, a thicker bilayer electrolyte may reduce the overall gravimetric energy density of the battery. Its assembly method involves cold-pressing one layer of electrolyte first, then cold-pressing another layer of electrolyte on its surface, by stacking the cathode, anode, and applying pressure together.
(3) In-situ generating a modification layer on the electrolyte surface (electrolyte/electrode interface). As shown in Fig. 3(c), Gao et al. added 1 mol/L LiTFSI DOL-DME electrolyte to the LGPS/Li interface, generating organic-inorganic hybrid lithium salts such as LiO-(CH₂O)ₙ-Li, LiF, -NSO₂-Li, and Li₂O, enabling the Li/LGPS/Li symmetric cell to stably cycle for 3000 hours under a current density of 0.1 mA/cm². Chien et al. used solid-state NMR imaging to find that significant Li depletion occurred at the interface of the Li/LGPS/Li symmetric cell after cycling, and coating with PEO-LiTFSI could improve the insufficient and uneven deposition of Li at the interface. Although these methods have improved the compatibility between sulfide electrolytes and metallic lithium anodes to some extent, they may also face issues such as the unclear principle of electrolyte dropping and reduced thermal stability of the electrolyte due to the addition of polymers.
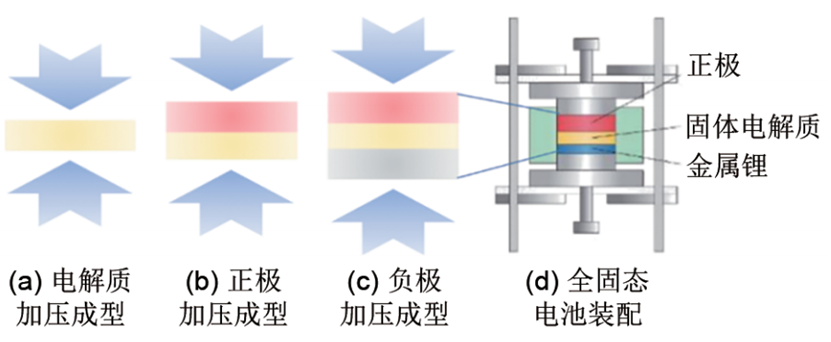
Figure 4: Assembly Method of All-Solid-State Lithium Batteries Based on Sulfide Solid Electrolytes
2.3 Assembly Method of All-Solid-State Lithium Batteries Based on Sulfide Solid Electrolytes
In terms of assembling all-solid-state lithium batteries with sulfide solid electrolytes, as shown in Fig. 4, the process mainly involves the following steps: ① Pressurizing and forming the electrolyte, typically with a pressure of 120–150 MPa; ② Pressurizing and forming the cathode, attaching a steel sheet as the current collector, typically with a pressure of 120–150 MPa; ③ Pressurizing and forming the anode: for metallic lithium, the typical pressure is 120–150 MPa; for graphite, the typical pressure is 250–350 MPa, with a steel sheet attached as the current collector; ④ Tightening the battery bolts. It should be noted that the reading on the hydraulic press dial should be converted based on the actual shape of the battery mold, and battery short-circuiting must be prevented during assembly.
References: Cui Yanming, Zhang Zhihua, Huang Yuanqiao, et al. Preparation and Assembly Methods of Electrodes for All-Solid-State Lithium Batteries[J]. Energy Storage Science and Technology, 2021, 10(3): 12.